3D reconstruction with electron density – DENSS in RAW¶
A new, exciting method for doing 3D shape reconstructions in SAXS yields actual electron density, rather than bead models. There are many potential advantages to this, but one significant one is easy handling of systems like RNA-Protein complexes or membrane proteins surrounded by lipids or detergents, which have more than one electron density. Bead models typically only have two (molecule and solvent) or three bead densities, and so typically fail to reconstruct these complex objects. DENSS has been fully implemented in RAW and will be used to reconstruct these electron densities.
Clear all of the data in RAW. Load the lysozyme.out file that you saved in the reconstruction_data folder in a previous part of the tutorial.
- Note: If you haven’t done the previous part of the tutorial, or forgot to save the results, you can find the lysozyme.out file in the reconstruction_data/lysozyme_complete folder.
Right click on the lysozyme.out item in the IFT list. Select the “Electron Density (DENSS)” option.
Running DENSS generates a lot of files. Click the “Select/Change Directory” button, make a new folder in the reconstruction_data directory called lysozyme_denss and select that folder.
Change the number of reconstructions to 5 and the mode to Fast.
- Note: It is generally recommended that you do at least 20 reconstructions. However, for the purposes of this tutorial, 5 are enough.
- Note: For final reconstructions for a paper, DENSS should be run in Slow mode. For this tutorial, or for obtaining an initial quick look at results, Fast mode is fine.
Click the “Start” button.
- Note: The status panel will show you the overall status of the reconstructions. You can look at the detailed status of each run by clicking the appropriate tab in the log panel.
Note that by default the densities are aligned and averaged, including enantiomer filtering, and a refined density is created from the average.
Wait for all of the DENSS runs and averaging to finish. Depending on the speed of your computer this could take a bit.
Once the reconstructions are finished, the window should automatically switch to the results tab. If it doesn’t, click on the results tab.
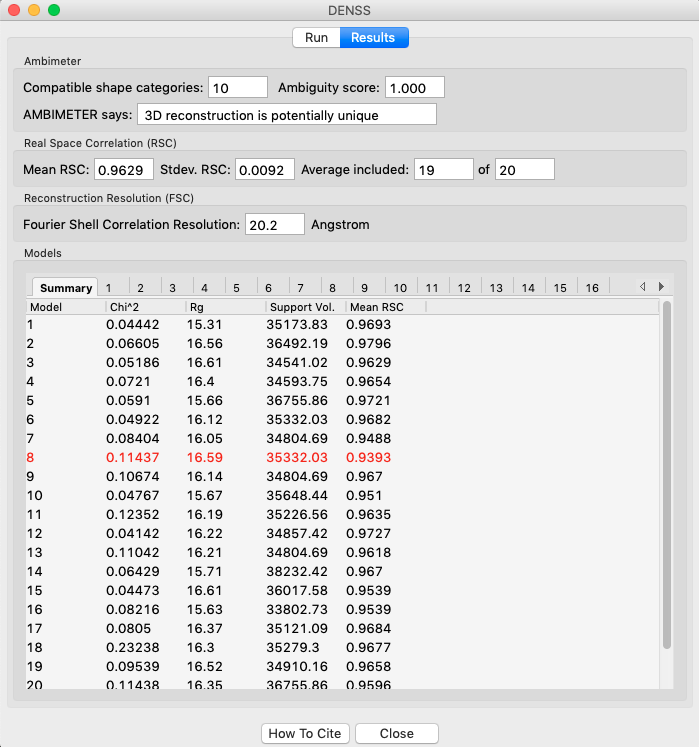
The results panel summarizes the results of the reconstruction runs. At the top of the panel there is the ambimeter evaluation of how ambiguous the reconstructions might be (see previous tutorial section). If averaging was run there is an estimate of the reconstruction resolution based on the Fourier shell correlation. In the models section there are several tabs. The summary tab shows the chi^2, Rg, support volume, and RSC to the reference model. If any model was not included in the averaging it is highlighted in red.
- Verify that the Rg is close to the expected value, and that the chi^2 and support volumes are relatively consistent between models.
- Note: Above we show the results for 20 runs instead of the 5 in the tutorial.
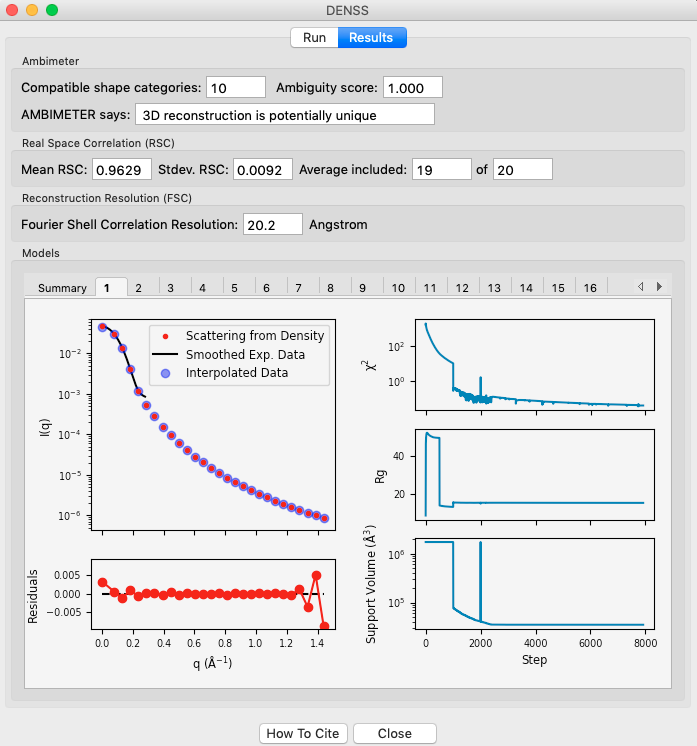
Individual model results are displayed in the numbered tabs. For each individual model there are plots of: the original data and the model data (scattering from density); the residual between the original data and the model data; and chi squared, Rg and support volume vs. refinement step.
- Verify that the residual between the actual data and the model data is small.
- Check that the chi squared, Rg, and support volume have all plateaued (converged) by the final steps.
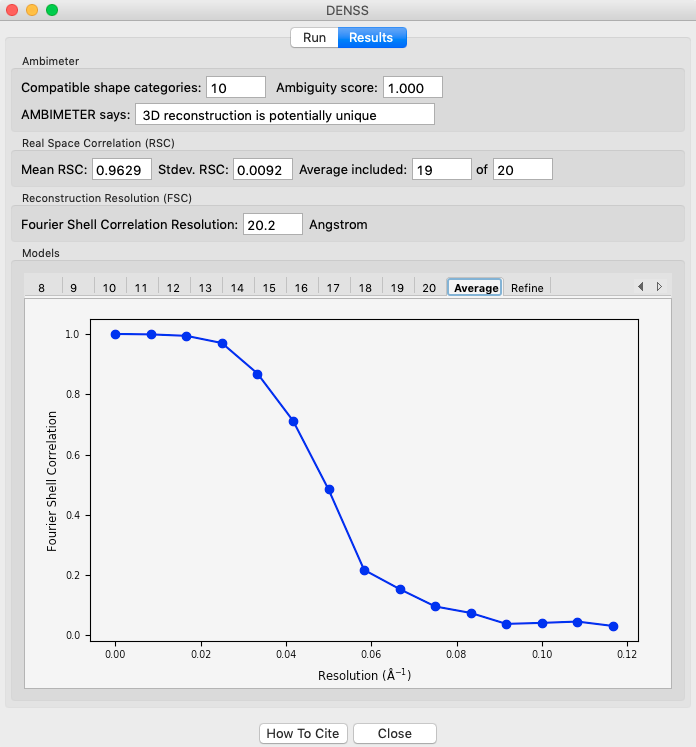
If the densities were averaged, the average tab will display the Fourier shell correlation vs. resolution.
- Note: The reconstruction resolution is taken as the resolution in angstroms where the correlation first crosses 0.5.
The results summary shown in Summary tab is automatically saved as a <prefix>_denss_results.csv csv file, e.g. for this data as lysozyme_denss_results.csv. All the plots shown on the individual model tabs are automatically saved as a multi-page pdf file with the same name.
Click the “Close” button when you are finished looking at the results and reconstructions.
The results from the individual DENSS runs are saved in the selected output folder as <prefix>_xx.mrc where xx corresponds to the run number: 01, 02, etc. For this tutorial that would be lysozyme_01.mrc, lysozyme_02.mrc, etc.
If averaging was done, final average density is saved in the selected output folder as <prefix>_aver.mrc. For this tutorial, that would be lysozyme_aver.mrc.
If refinement was done, the final refined density is saved in the selected output folder as <prefix>_refine.mrc. For this tutorial that would be lysozyme_refine.mrc.
Note: .mrc files can be opened in Chimera and pyMOL. For tips about how to visualize the density and align it with known structures see the appropriate sections here: http://www.tdgrant.com/denss/tips/.